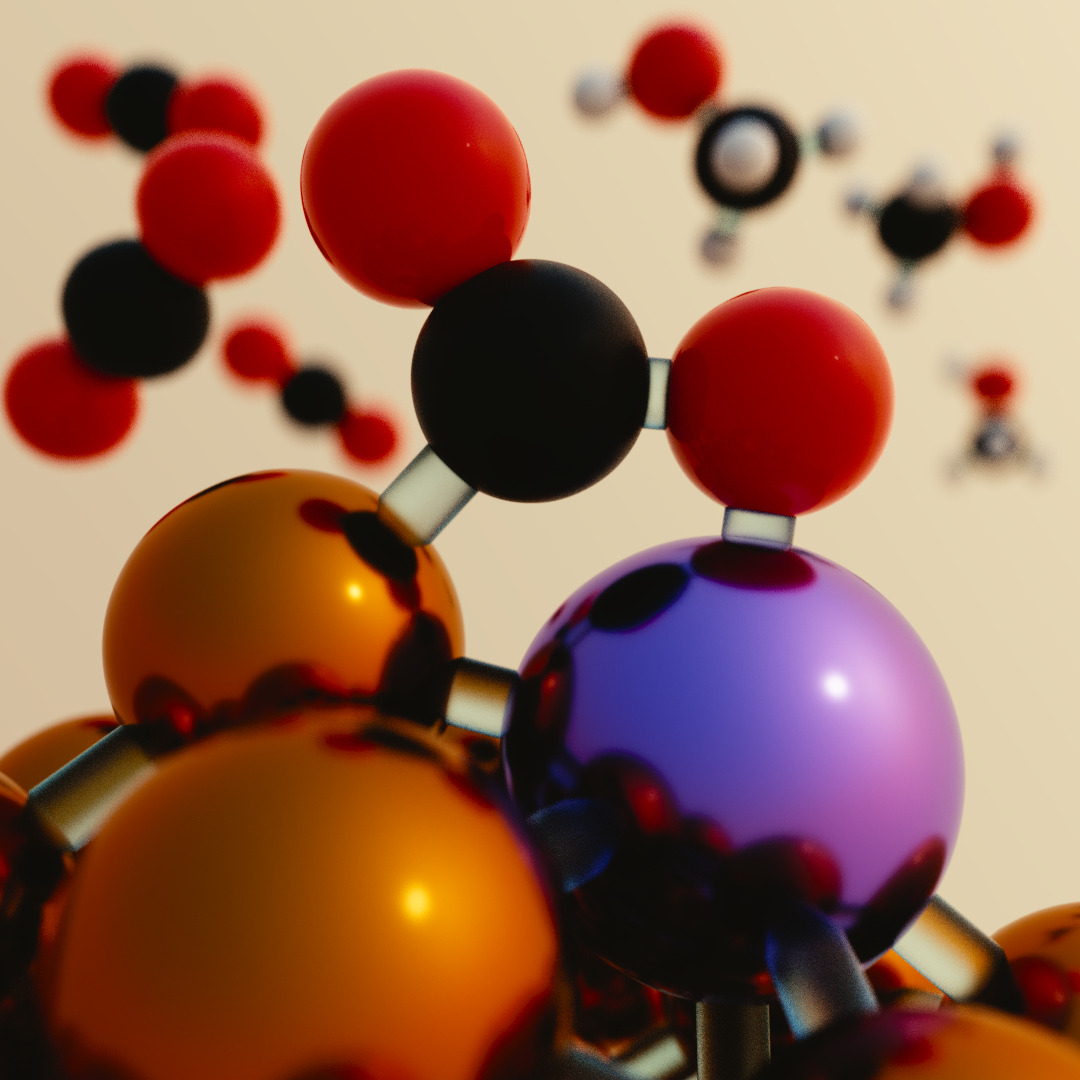
Nanocatalysis
Catalysis becomes extremely complex at nanoscale. The goal of this work is to understand
the catalytic behavior of metal-oxide supported nanoparticles under realistic experimental
conditions. We elucidate the bonding characteristics of adsorbates on nanoparticles and
develop relationships predicting their binding energy versus the nanoparticle structural
characteristics. Additionally, we investigate the catalytic mechanisms on both metals and
metal oxide supports by taking into account complex physical phenomena (support effects and
reconstruction) occurring on the catalyst. Finally, we propose novel nanocatalysts with
optimal catalytic activity under experimental conditions. Applications include enviromental
catalysis and mitigation of greenhouse gases concentration.

Biomass Conversion
Dehydration reactions are the most important reactions for converting biomass to fuels and
chemicals. A fundamental understanding of the dehydration mechanisms can help us elucidate
and eventually control the selective dehydration of complicated biomass molecules, such as
polyols, to value-added chemicals. In this work, we investigate the dehydration of simple
alcohols on various metal-oxides in the presence of water. We develop dehydration
relationships as a function of the metal-oxide acidity and the alcohols properties, aiming
to predict the dehydration behavior of polyols on different oxides.
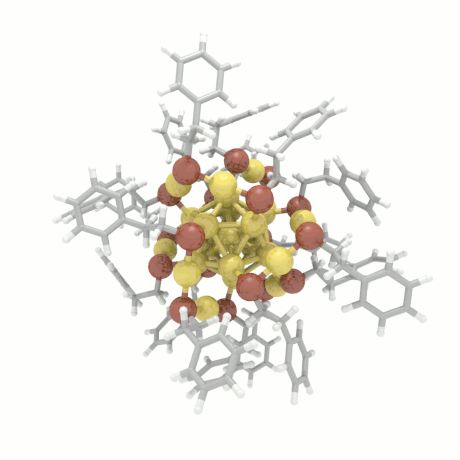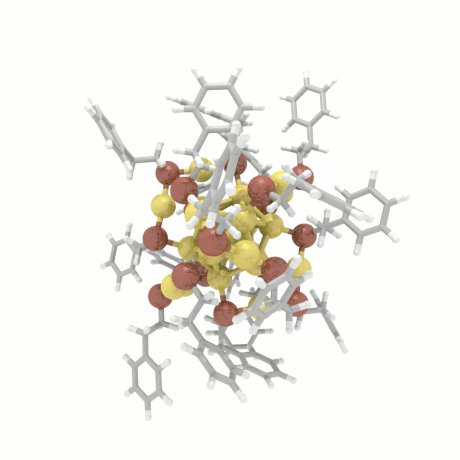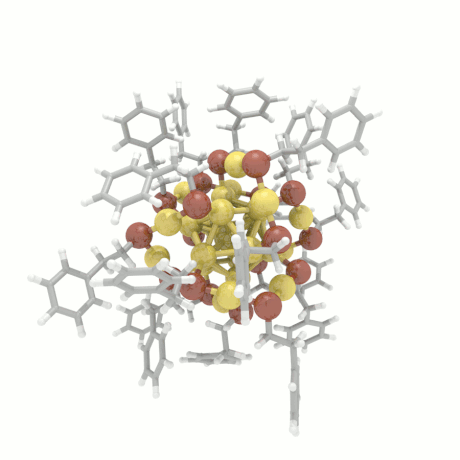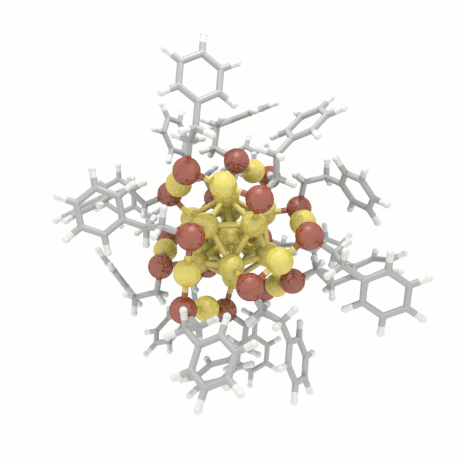
Nanoparticle Growth
The nanoparticle properties are directly related to their structural characteristics. Even
though nanoparticles of different sizes and morphologies can be synthesized in the lab, their
growth mechanisms are completely unknown. Here, we investigate the colloidal nanoparticle
growth in the presence of solvents and capping agents. We provide insights into the
nanoparticle growth mechanisms and propose design guidelines to control nanoparticle
characteristics (size, shape, dispersity) during synthesis.
Hydrogen Storage (prior research area)
We provide a firm understanding on how the structural (curvature and chirality) and electronic
(point charges) characteristics of SiC, BN and alkali doped C nanotubes affect the physisorption
energy of molecular hydrogen. Based on this, we design novel synthetic pathways to increase
hydrogen storage in nanomaterials.


